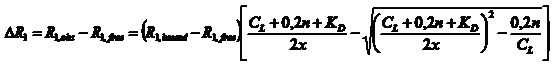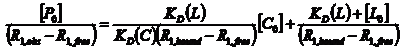|
INCDTIM |




|
Results: 2011 2012 2013 2014 2015 2016
2015
Competitive NMR study of the interaction of 5 – fluorouracil and cytarabine with HSA
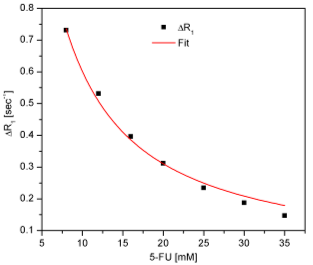
a) 5-fluorouracil – human serum albumin interaction 5-fluorouracil is an important and widely used antineoplastic drug that is carried in the serum by plasma proteins. Protein binding studies of this drug to human serum albumin (HSA) have been carried out especially by fluorescence spectroscopy and circular dichroism. These studies were conducted in order to determine the dissociation constant for binding sites with high affinity. To gain quantitative information concerning the number of low-affinity binding sites on HSA and the dissociation constant of the formed macromolecular complex, we performed a NMR study based on the selective relaxation rate variation of the 5-fluorouracil H(1) proton as a function of its concentration at a constant human serum albumin concentration (0,2 mM). We have prepared a set of 7 samples with 5-fluorouracil concentration in excess to HSA (between 8 and 35 mM). For a pure 5-fluorouracil sample we have obtained the selective relaxation rate value of R1,free = 0,0978 ± 0,00003 sec-1. The experimentally obtained results, presented in Fig.1, were fitted with the equation:
Fig.1. Plot of the observed (DR1=R1,obs – R1,free) selective relaxation rates of H(1) 5-fluorouracil proton as a function of its concentration . The obtained results are:
R1,bound = 1,871 sec-1 n = 17,9 ± 0,3 KD = 0,372 mM r2 = 0,991
b) Cytarabine – human serum albumin interaction
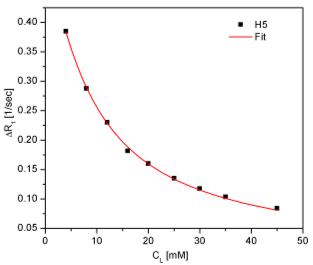
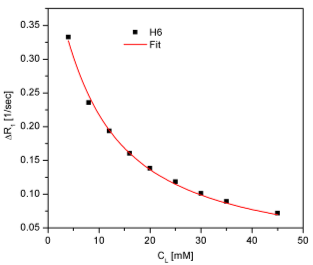
Cytarabine or cytosine arabinoside is a chemotherapy agent used mainly in the treatment of cancers of white blood cells such as acute myeloid leukemia (AML) and non-Hodkin lymphoma. It is also known as ara-C (arabinofuranosyl cytidine). It kills cancer cells by interfering with DNA synthesis. Information regarding its capacity to bind serum and tissue proteins is invaluable in ascertaining bioavailability which is determined by the amount of free drug present in the serum. From fluorescence studies, it is found that cytarabine associates with HSA with an association constant of Ka = 22·103 M-1 and with BSA with Ka = 22·103 M-1. In both cases the stoichiometry was supposed to be 1:1 (n = 1), typically for binding sites with high affinity. In order to gain information about the number of non-specific binding sites with low affinity on HSA, as well as the dissociation constant of the cytarabine : HSA complex, we performed a NMR study based on the selective relaxation rate variation of some cytarabine protons as a function of its concentration at a constant human serum albumin concentration (0,2 mM). The investigated protons were H(5) and H(6). The chemical structure and the proton notations can be found in the Scientific Report 2015.
Fig.2 The observed selective relaxation rates versus [Cyt] for H(5) si H(6) protons of cytarabine For a free cytarabine sample (10 mM), we obtained:
R1,free(H5) = 0,322 ± 0,001 sec-1 R1,free(H6) = 0,373±0,001 sec-1
The experimentally obtained results, presented in Fig.2, were fitted with the eq. (1) and the results are:
Because the obtained values for H(5) and H(6) are very close, we consider that the cytarabine interaction with HSA can be characterized by an average value of the number of binding sites ,n, and the dissociation constant, KD, namely:
n = 13,05 ± 0,25 KD = 5,06·± 0,23 mM
c) 5- flourouracil – cytarabine – human serum albumin interaction
An NMR investigation of this ternary system was used to describe quantitatively the competitive binding between 5-fluorouracil and cytarabine with human serum albumin. Having common binding sites (subdomain IIA), by following the selective relaxation rates variation of one ligand L (3-Fu) proton, as a function of the competitor ligand C (Cyt) concentration, we can determine the dissociation constant that characterize the competitor ligand C, in the presence of L. To realize such an experiment, first it is necessarily to dete4rmine in a non-competitive binding experiment the following parameters: KD(L), R1,free (L) and R1,bound(L). In a second experiment, we keep constant the ligand L and human serum albumin concentrations, for different competitor C, concentrations. In this way, it is possible to determine KD(C) in the presence of ligand L. In our particular case, the first experiment consisted in the determination of R1,free(5-Fu), R1,bound(5-Fu) and KD(5-Fu). The obtained results were presented in section (a) . For the second experiment we prepared a set of 7 samples in which:
[hsa] = [P0] = 0.2 mM = ct. [L0] = [5-Fu] = 10 mM = ct. [C0] = [Cyt] varying between 4mM si 30 mM
In the Scientific Report 2015, we deduced the theoretical expression for the selective relaxation rate variation of L0 = 5-Fu, as a function of the competitive ligand C0 = Cyt., at a constant concentration of HSA. Working with both ligands concentration in excess we obtained:
A plot of [P0]/ (R1,obs – R1,free) as a function of [C0] has a slope m =
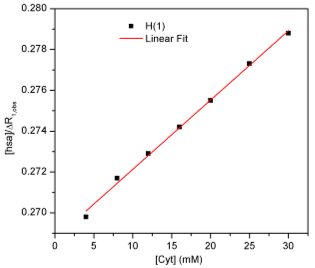
Fig.3. Plot of the 5-Fu H(1) proton relaxation data according to Eq.(2) for competitive titration with cytarabine
Fitting the experimental data with a linear equation (Y = mx + n) gave the following results:
m = 3,3946·10-4 sec n = 0,2687 mM·sec r2 = 0,9964
From the value obtained for m, we deduced the dissociation constant of cytarabine, in the presence of 5-fluorouracil : KD(cyt) = 616,7 mM
In the case of direct binding experiments (section a and b), the obtained results for Ka = 1/KD were:
Ka(5-Fu) = 2688,2 M-1 Ka(cyt) = 197,6 M-1
The conclusion in the case of these two ligands is that 5-fluorouracil is much stronger bound to human serum albumin than cytarabine. In a competitive binding, in the presence of 5-fluorouracil, cytarabine binds very weakly to HSA, its dissociation constant being almost two order of magnitude bigger then the value which characterize the direct binding. Due to the fact that both ligands bounds in subdomain IIA of HSA, we conclude that cytarabine is not able to replace 5-fluorouracil from its binding sites, only to a very small extent. |