Scientific Report
Period: January - December 2023
O3 - |
The photochemistry of polydopamine-functionalized graphene and TiO2 nanostructures; |
M04 - |
Task 4: Mapping the photochemical behavior of PDA coated graphene nanostructures using combined experimental and theoretical investigations; "D(29)" |
M05 - |
Task 5: Mapping the photochemical behavior of PDA coated TiO2 nanostructures using combined experimental and theoretical investigations; "D(34)" |
M06 - |
Drawing general conclusions about the photochemical behavior of PDA coated graphene and TiO2 nanostructures, as well as project dissemination (Conference participation, progress report, scientific article); "D(36)" |
Experimental preparation of graphene surfaces: The graphene derivatives used - graphene oxide (GO) and thermally reduced graphene oxide (trGO) - were prepared by optimized synthesis methods in INCDTIM, with morphologically and structurally reproducible results. Namely: graphene oxide was prepared according to a Hummers procedure modified by introducing a graphite pre-oxidation step, which is extremely necessary to ensure complete oxidation of the starting material.
Experimental preparation of adsorbed polydopamine on graphene and TiO2 substrate: In order to study the optical properties of polydopamine on different substrates e.g. TiO2 , graphene, reduced graphene, we considered several methods of dopamine polymerization. In this approach we proposed 3 synthetic methods: i) classical polymerization in 10 mM TrisCl buffer, pH=8.5, ii) polymerization in the presence of 10 mM NaOH and iii) polymerization in water. For each process a mass ratio of 1:1, nanoparticles to dopamine, with a concentration of 2mg/ml in each solution were used.
UV-Vis absorption-emission spectra of polydopamine adsorbed on graphene and TiO2 substrate: The optical properties of dopamine-coated TiO2 nanoparticles at different time intervals were investigated. The spectra obtained are shown in Figure 1 and show a decrease in the characteristic bandwidth of TiO2 nanoparticles over time, suggesting dopamine coating. In contrast, in the case of coating TiO2 nanoparticles with PDA, the following changes were observed, depending on the PDA mass ratio: TiO2 nanoparticles. Thus at a ratio of 0.5:1, the compound shows the absorption band characteristic of TiO2 nanoparticles, with a shoulder at 280 nm, characteristic of PDA. Increasing the ratio to 1:1, i.e. 1.5:1, the band at 280 nm shifts to 290 nm and the band at 317 nm characteristic of PDA increases. The characteristic optical properties of the PDA coated surfaces were investigated by UV-Vis absorption spectroscopy. The absorption spectrum of TiO2 nanoparticles of 32 nm diameter shows a broad band covering the UV and visible range, with a maximum at 349 nm. TiO2 nanoparticles coated with PDA obtained from polymerization in the presence of NaOH (PDA-NaOH) show a modified absorption, indicating a maximum at 322 nm and one at 290 nm. The spectrum is similar to the optical response characteristic of TiO2 nanoparticles coated with PDA obtained by classical polymerization in TrisCl buffer (PDA-Tris). In contrast, TiO2 nanoparticles coated with PDA obtained by polymerization in water (PDA-water) show an absorption band similar to TiO2 nanoparticles and shifted towards blue at 336 nm. A low intensity band is also observed around 270 nm.
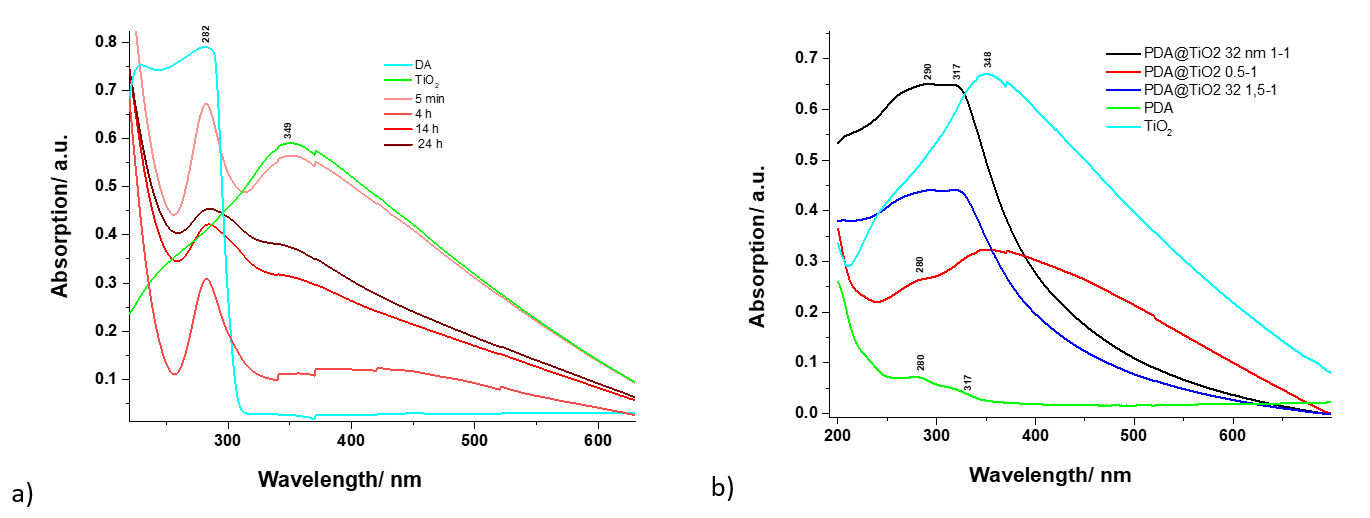
Figure 1. UV-Vis absorption spectra of dopamine-coated TiO2 nanoparticles recorded at different time points (a) and characteristic of polydopamine deposited on TiO2 nanoparticles (b).
PDA-NaOH-coated graphene and PDA-apa-coated graphene, respectively, showed an absorption band centered around 250 nm, while PDA-Tris-coated graphene showed two bands at 283 and 309 nm. In the case of the reduced graphene coated with the three types of PDA, the characteristic absorbance of each of the three compounds was much reduced compared to the previously presented compounds. Reduced graphene coated with PDA-apa showed the highest absorption, with a band at 277 nm. The reduced graphene coated with PDA-NaOH showed a band at 268 nm and the reduced graphene coated with PDA-Tris showed a band at 245 nm.
Fluorescence spectroscopy has also been used to investigate different types of dopamine-coated surfaces, namely polydopamine, but no fluorescence spectra were recorded for these samples. This result suggests that coating surfaces such as TiO2 nanoparticles or graphene with PDA leads to quenching of the weak fluorescence signal characteristic of some PDA samples, such as that obtained by classical polymerization in TrisCl buffer.
Theoretical modeling of the dopamine molecule adsorbed on the graphene-like substrate: The equilibrium geometries of dopamine and dopamine-quinone molecules adsorbed on the graphene-like substrate and graphene oxide were determined by the density functional theory (DFT) method using the ωB97X exchange-correlation functional [1] with empirical correction for dispersion in Grimme's D3 form [2] using the def2-TZVPP basis set [3]. The RIJCOSX approximation [4], designed to speed up Hartree-Fock and DFT calculations with hybrid exchange-correlation ional functions, was considered, together with the auxiliary basis set Def2/J [5] for Coulomb-type component factorization. The mentioned theoretical methods and numerical approximations are implemented in the ORCA software suite [6-8].
The first molecular complex analysed was constructed using a 7x3 polycyclic aromatic ring network representing the graphene substrate, i.e. the dopamine molecule and its quinone form. The spatial shape can be seen in Figures 2a and 2b. As can be seen in Figure 2, the dopamine and dopamine-quinone molecules lie in a plane parallel to the aromatic surface and the mobile fragment -CH -CH -NH2 will also remain attached to the surface. The interatomic distance between the planes of the molecules and the surface is in the range of 3.20 - 3.50 Å in the case of dopamine and 3.13 - 3.50 Å in the case of dopamin-quinone. After determining the geometrical positions, UV-Vis absorption spectra were determined using the time-dependent DFT method (TD-DFT/ωB97X-D3/def2-TZVPP). The theoretical spectra are shown in Figure 3. In the spectral region 350 - 700 nm, the absorption spectrum is dominated by the graphene contribution showing an intense absorption around 537 nm. This absorption peak is due to the inhomogeneity of the aromatic ring structures of the surface, whereby the C=C double bonds are weakened by excitation. The spectral fingerprints of dopamine and dopamin-quinone can be observed in the spectral range 200 - 350 nm, even though dopamin-quinone has excited electronic states also in the range 350 - 530 nm, but the intensity of these states are very weak and they do not appear in the absorption spectrum. In order to understand more deeply the physical phenomena that occur following the excitation of electronic states, we analyzed the distribution of electronic charges induced by the excitation. Even in the ground state, due to the intermolecular interaction between the molecule and the substrate, there is charge migration between the two. Thus, in the case of dopamine we observe a transfer of 0.068e from dopamine to substrate, while in the case of dopamine-quinone this value is lower, only 0.031e.
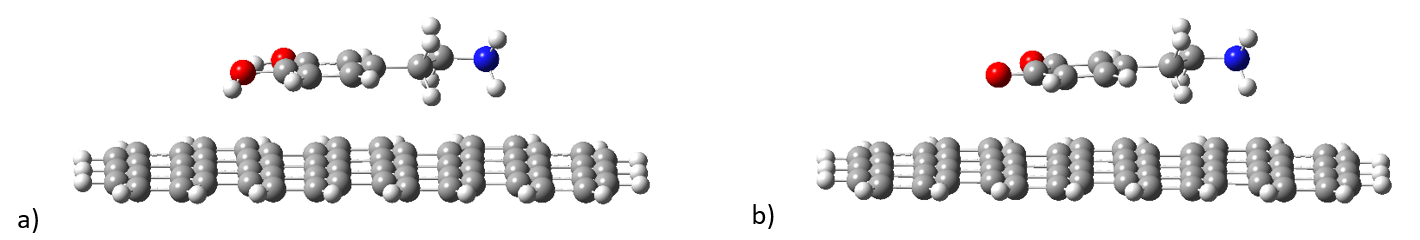
Figure 2. Geometric configuration of dopamine and dopamine-quinone adsorption on graphene-like surfaces.
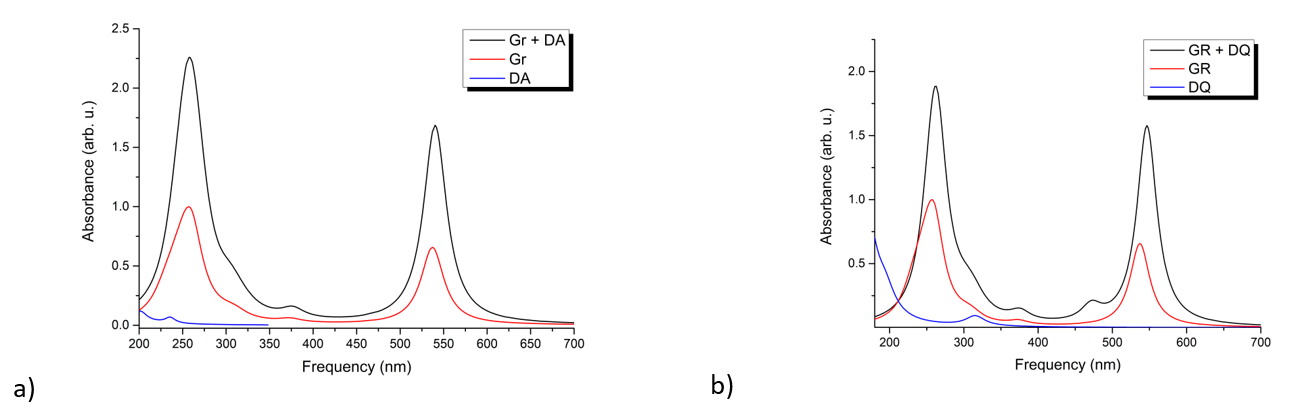
Figure 3. UV-Vis absorption spectra for the molecular complexes: a) dopamine + graphene, respectively b) dopamine-quinone + graphene.
Stage summary:
✔
Graphene, graphene oxide and thermally reduced graphene oxide surfaces were prepared by experimental techniques using the method optimized in INCDTIM, and the spectral characteristics of these surfaces were revealed by RAMAN spectroscopy;
✔
Structures of polydopamine adsorbed on different graphene surfaces, i.e. TiO2, were prepared by experimental techniques;;
✔
UV-Vis absorption-emission spectra of adsorbed polidopamine in three different solvent media were measured. The characteristic UV-Vis absorption spectra span the spectral range 200-900 nm, showing an increase in absorbance at 288, 230 and 208 nm, and they remain the same with decreasing solution concentrations;
✔
UV-Vis absorption-emission spectra of polydopamine adsorbed on graphene and TiO2 substrate in three different solvent media were measured. The characteristic UV-Vis absorption spectra show significant changes in the spectral range 200 - 350 nm caused by the different solvent media used in the preparation. No fluorescence spectra were recorded suggesting that coating of TiO2 nanoparticle or graphene surfaces with PDA leads to quenching of the weak fluorescence signal characteristic of some PDA samples;
✔
DFT and TDDFT theoretical calculations were used to obtain the geometries of the binary molecule-surface structures and determine their UV-Vis absorption spectra. It was observed that in addition to the electronic transitions located on molecules or surfaces, charge transfer transitions occur which are characteristic only of binary structures. It has been shown that dopamine and dopamine-quinone molecules behave differently in terms of charge transfer phenomena, in the former case we have a transfer from the molecule to the surface, and in the latter case the transfer shows a totally opposite direction. It has been shown that relaxation of the electronic states of the molecule on the surface reduces the intensity of charge transfer;
Bibliography:
J.-D. Chai and M. Head-Gordon, The Journal of Chemical Physics, 128, 084106, 2008.
S. Grimme, J. Antony, S. Ehrlich and H. Krieg, The Journal of Chemical Physics, 132, 154104, 2010; Y.-S. Lin, G.-D. Li, S.-P. Mao and J.-D. Chai, Journal of Chemical Theory and Computation, 9, 263-272, 2013.
F. Weigend and R. Ahlrichs, Physical Chemistry Chemical Physics, 7, 3297-3305, 2005.
F. Neese, F. Wennmohs, A. Hansen, U. Becker, Chemical Physics, 356, 98-109, 2009.
F. Weigend, Physical Chemistry Chemical Physics, 8, 1057 - 1065, 2006.
F. Neese, WIREs Computational Molecular Science, 8, e1327, 2018.
F. Neese, F. Wennmohs, U. Becker, C. Riplinger, Journal of Chemical Physics, 152, 224108, 2020.
F. Neese, WIREs Computational Molecular Science, 12, e1606, 2022.