MSH2: Universal Multiscale Simulations for Hydrogen Storage in Novel Materials

Host institution: National Institute for Research and Development of Isotopic and Molecular Technologies
Program: PN II – HUMAN RESOURCES
Type of the project: YOUNG RESEARCH TEAMS
Project code: PN-II-RU-TE-2014-4-1309
Project duration: 01.10.2015 — 30.09.2017
Principal Investigator: Liviu Zarbo (CV)
Phone: (+4)0264-584037, ext. 196
Email: liviu.zarbo@itim-cj.ro
| # | Members of the implementation team | Tasks within the project |
|---|---|---|
| 1 | Dr. Daniel Bilc | theory |
| 2 | Dr. Liviu Zarbo | theory |
| 3 | Dr. Ioana Grosu | synthesis and characterization |
| 4 | Dr. Gabriela Blanita | synthesis and characterization (adsorption) |
| 5 | Dr. Eng. Ioan Coldea | engineering |
| 6 | Dr. Xenia Filip | theory and characterization (NMR) |
| 7 | PhD candidate Maria Miclaus | X-ray characterization |
| 8 | PhD candidate Marius Oancea | theory |
| 9 | Postdoctoral Researcher Dr. Mirabela Golban | synthesis of linkers for MOFs |
| Proposal abstract | The realization of efficient hydrogen storage in materials will have a tremendous impact on the fields of renewable energy and clean transportation. The most promising high uptake materials are based on the physisorbtion of the hydrogen which offers fast, reversible, energetically cheap storage. However, materials synthesized so far have poor ambient conditions performance due to the weak forces binding the molecular hydrogen. We will develop a universal multiscale simulation tool to aid the design of better hydrogen storage materials that will improve upon the state of the art simulations as follows. i) The polarization effects in electric fields will be included both at microscopic and macroscopic scales. ii) The weak interactions involved in the physisorbtion will be correctly treated at the ab initio level, thus revealing the most favorable microscopic features of the materials for hydrogen storage and ensuring the transferability of the code. iii) The simulation will be precise and transferable allowing in silico screening on a large class or materials, thus greatly reducing the experimental overhead. We aim to open the new research direction of electrically controlled hydrogen storage. We will design and then synthesize in the lab polarizable nanoporous materials with electrically tunable hydrogen storage properties based on covalent organic frameworks. We will assess the potential of these materials for industrial applications. |
| Phase 1: 01.10.2015--31.12.2015 |
Obj. 1.1 SAPT-DFT simulations for binding centers and force
fields. Report summary. |
| Phase 2: 01.01.2016--31.12.2016 |
Obj. 2.1 SAPT-DFT simulations for binding centers and force
fields (cont from 2015). Obj. 2.2 Implementation of polarization effects in SAPT-DFT. Obj. 2.3 GCMC simulations. |
| Phase 3: 01.01.2017--30.09.2017 |
Obj. 3.1 Computer design of novel materials. Obj. 3.2 Hydrogen storage experiments in novel materials. |
The following table summarizes our work for Phase 1 and 2. We merged the presentations for the tasks that started in 2015 and ended in 2016.
| Phase 1+Phase2 + Phase 3 |
|||
| Objective | Task description | Report summary | Results |
| Objective 1.1 and 2.1 SAPT-DFT simulations for binding centers and force fields. | 1.1.1 and 2.1.1 The implementation of DFT-SAPT for computing force fields in the absence of electric fields. | We chose a number of systems to test the methodology for
computing force fields in the absence of electric fields. We
tested the computation of localized charges and multipoles as
well as the parametrization of force fields on simple molecules
and on molecular models chosen for isoreticular MOFs. Most of
this work was done using the CamCASP software which correctly
implements DFT-SAPT. |
2.1 Methodology for determining force fields and binding
centers. 2.1.1 Conference poster presentation: |
| Objective 2.2 Implementation of polarization effects in DFT-SAPT. | 2.2.1 Implementation of polarization effects in DFT-SAPT. Testing results with MP2 or CC. | We introduced the polarization effects by analyzing the entire
MOF cell in the presence of electric fields. The polarization of
the MOF structure was analyzed at DFT level. We saw that under
the effect of electric fields, the atomic positions get
modified, resulting in an overall polarization of the MOF
structure. We determined how the binding energies of hydrogen (and methane) change as we apply electric field on MOFs. For relatively large fields (~5GV/m) we see a 5-10% increase in binding energies both on the linkers and near the coordination sites. Our results for methane have been presented at College on Multiscale Computational Modeling of Materials for Energy Applications, Trieste, Italy (2016) Here is the poster presentation. Liviu Zarbo gave a talk at INCDTIM on our work on electrically controlled adsorption. See the talk here. |
2.2 Methodology for implementing the polarization effects in
DFT-SAPT. 1 Poster presentation |
| Objective 2.3 Setup the GCMC simulations. | 2.3.1 Our GCMC simulations will follow the standard methodology and use transferable force fields with physically motivated terms. Polarization effects will be included in those simulations. | We setup the standard methodology for adsorption simulations.
We used the RASPA software whose libraries of force fields,
structures and molecules are easily modifiable. Using our own
structures and force fields, we see that for a polarized
IRMOF-1, the adsorption isotherms show an increase in of uptake
in electric field. This is consistent with the increased binding
energies obtained at the previous activity. |
2.3 GCMC methodology for adsorption simulations in electric fields. |
| Objective 3.1 Computer aided design of novel materials. | 3.1.1 This step will be possible once our multiscale simulation tool is fully tested. From our simulations on known materials, we plan to infer some rules of thumb for maximizing hydrogen storage in the presence of external electric fields. We will perform in silico experiments on polarizable storage materials. The best material(s) will be synthesized in the lab. | We used a polarized MOF model to compute how the hydrogen
adsorption capacity changes with the polarizability of MOF. We
showed that an increase in polarizability can more than double
the uptake for a polarizable MOF. We had two conference talks regarding our results concerning polarization mediated binding in MOFs. EMR 2017 conference in Lisbon, Portugal Here is the presentation. Nanotech 2017, Paris, France. Here is the talk. |
2 presentations at ISI indexed conferences. |
| Objective 3.2 Hydrogen uptake experiments on novel materials. | 3.2.1 Depending on the results provided by theoretical calculations (Milestone 4) new boroxine and boronate ester based COF systems with 3D topology will be designed and synthesized in order to produce materials which possess enhanced hydrogen storage capacities by employing building blocks that are decorated with suitable units with large dipole moments (e. g. dipolar aromatic systems or azulene). Porous materials based on the ionic linkage between carboxyl and amidine groups will be also designed, synthesized and investigated. | We synthesized 2 polarizable MOF compounds. We also built a modified reactor for adsorption measurements in electric field. So far, we performed measurements on typical MOFs such as MIL-101. The modified reactor gives correct adsorption isotherms. | Synthesis of 2 polarizable MOFs for hydrogen adsorption. |
First Isotherms of adsorption in electric field for IRMOF-1 compared with the ones in 0 electric field.
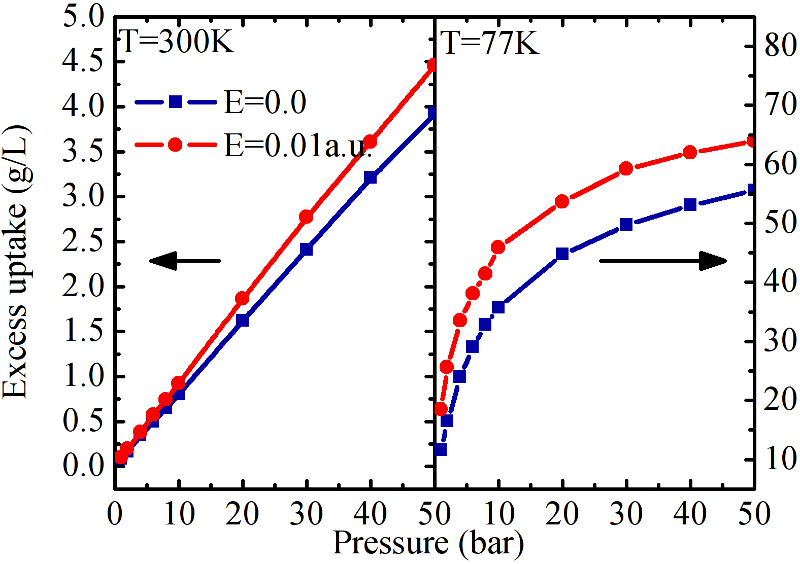
Effect of polarizability of MOF on their electric field uptake. For large polarizability, the uptake can more than double at room temperature.
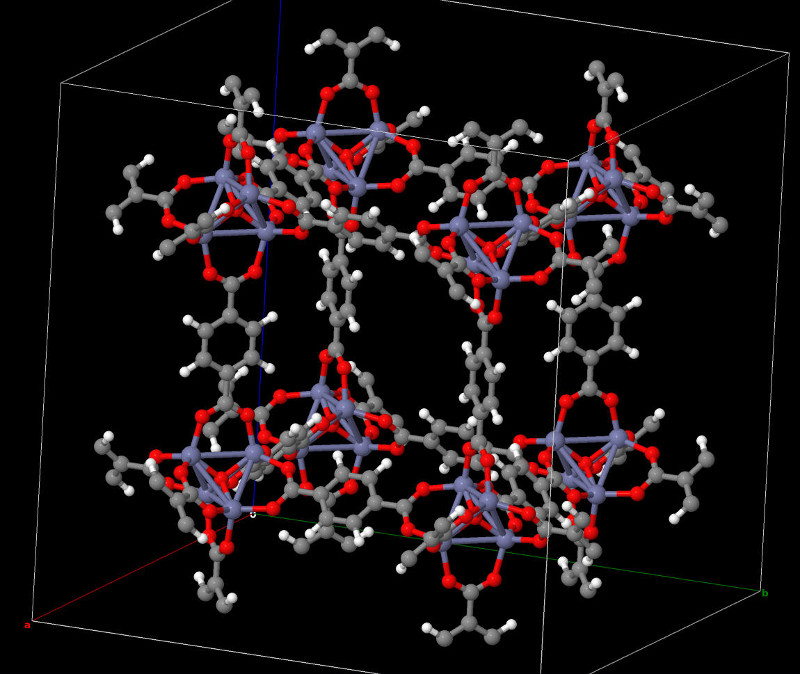
Atomic structure of a polarizable MOFs synthesized in this project and X-Ray diffractogram.
Reactor for electric field adsorption measurements.
Electric field profile inside the reactor -- insulation is done with teflon.
Adsorption isotherms measured for MIL-101 using our reactor.
