Scientific Report
Period: January - December 2019
O2 - |
Searching for different ligand structures in order to get high efficient and well-controlled "Low-spin" and "High-spin" transitions; (Additional study) |
M06 - |
Drawing general conclusions about the spin crossover performance of different macrocycles with respect of the used molecular fragments; (Additional study) |
1. Reversibility and structural stability of the proposed SCO complexes
The wide range of possibilities for choosing the corresponding metal ions, organic ligands or the coordination configuration give us various solutions for designing organometallic complexes with desired properties through which it is possible to improve the efficiency of the spin crossing, photocatalytic activity or structural thermal expansion1-4. The ground state electronic structures of the metal-organic complexes are mainly defined by the charge transfer effects from the electron reach ligand systems to the central positive metal ions5-7.
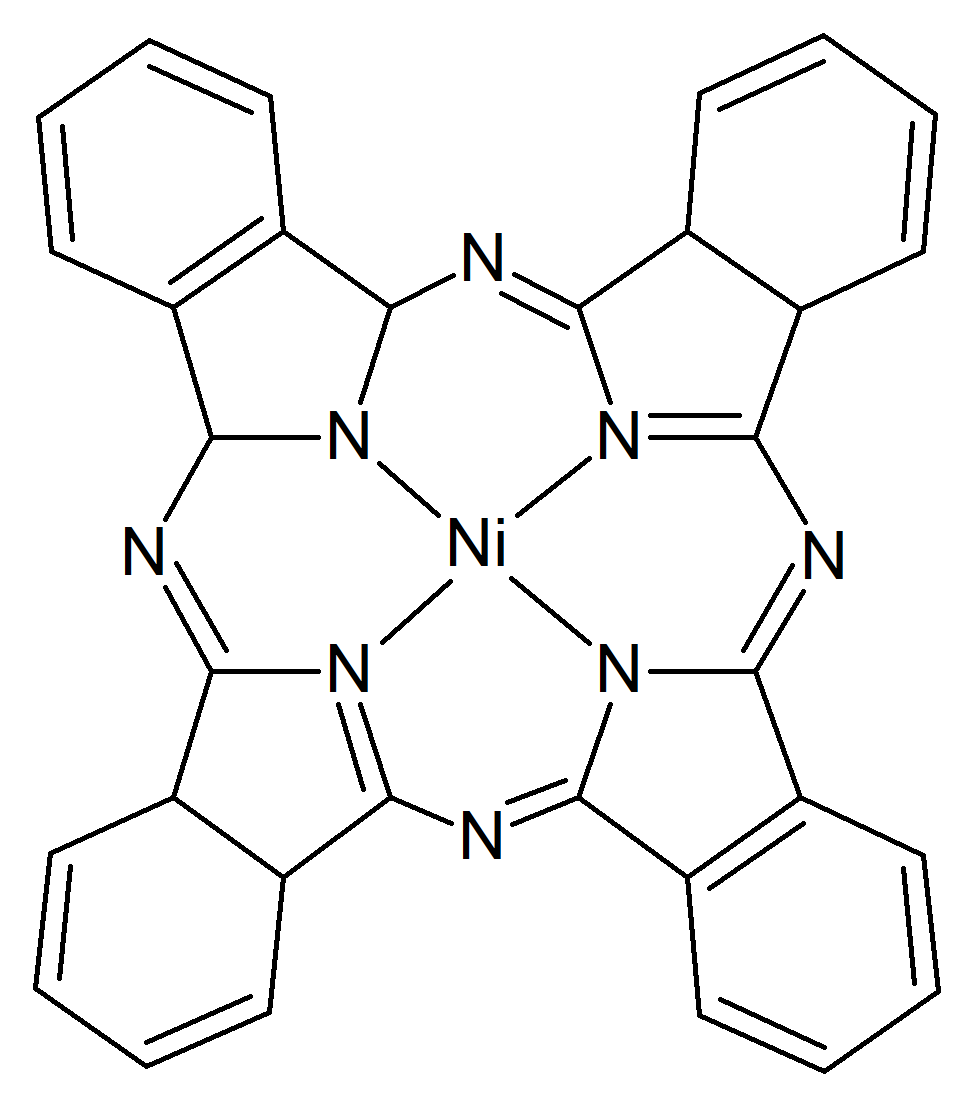
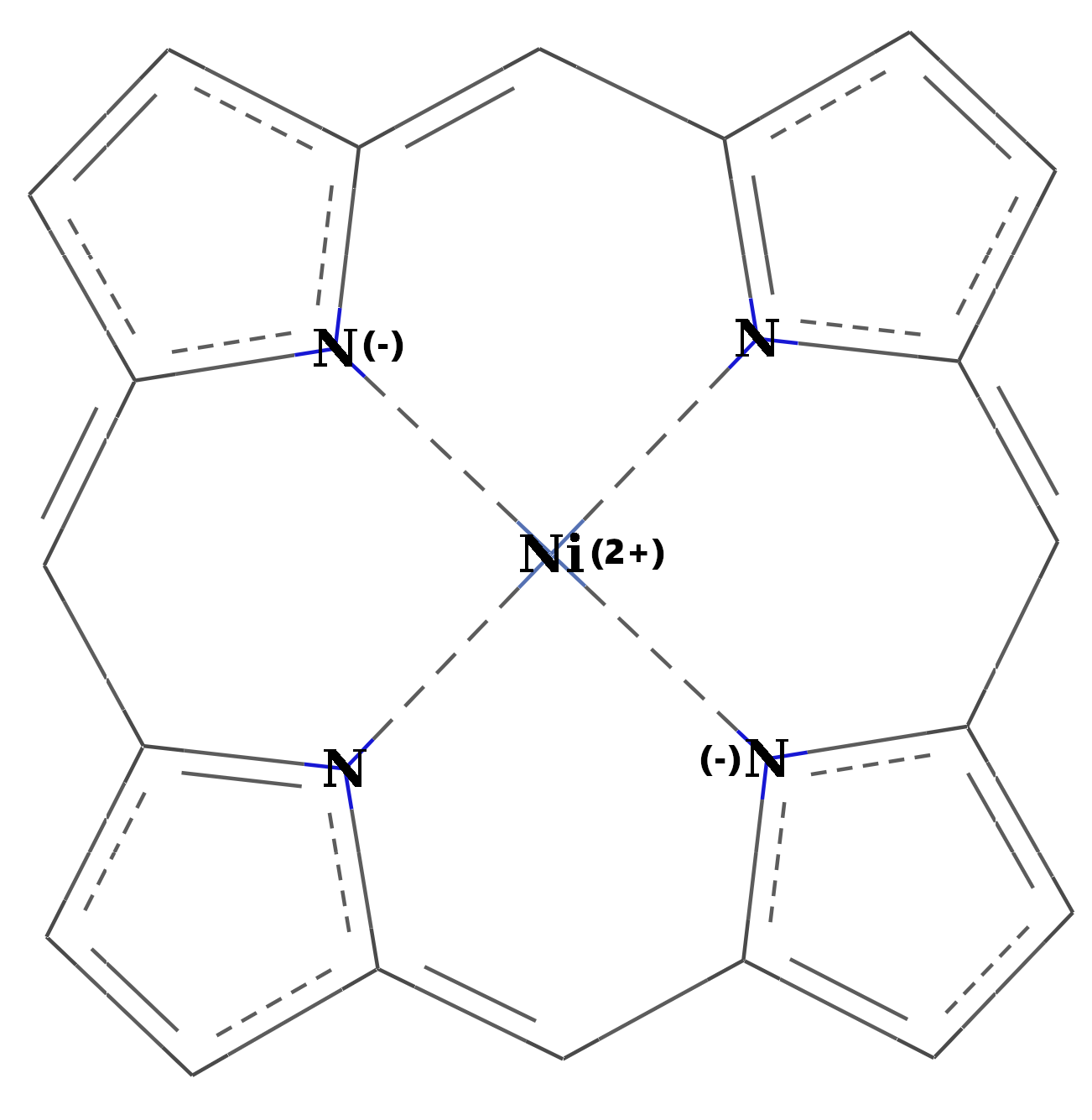
We have proposed to investigate the role of the charge transfer effects on the structural stability and the spin transition mechanism in organometallic complexes. Accordingly, structures with two different, square-pyramidal and octahedral ligand-metal coordination have been selected, wherein the structure of the ligands have been also changed. For this propose, porphyrin (P) and diketo-pyrphyrin (DP) macrocycles were used as four-coordination planar ligands (See Figure 1), while pyridine (Pi), (or pyrrole (Py)) and mesylate (Me, in its neutral CH3-SO2OH and anion CH3-SO3¯ forms) molecular groups have been considered as possible vertical ligands (See Figure 2).
(a)
(b)
Figure 1. The two geometry configurations of the central Ni(II) ion bounded by (a) porphyrin and (b) diketo-pyrphyrin planar organic ligands.
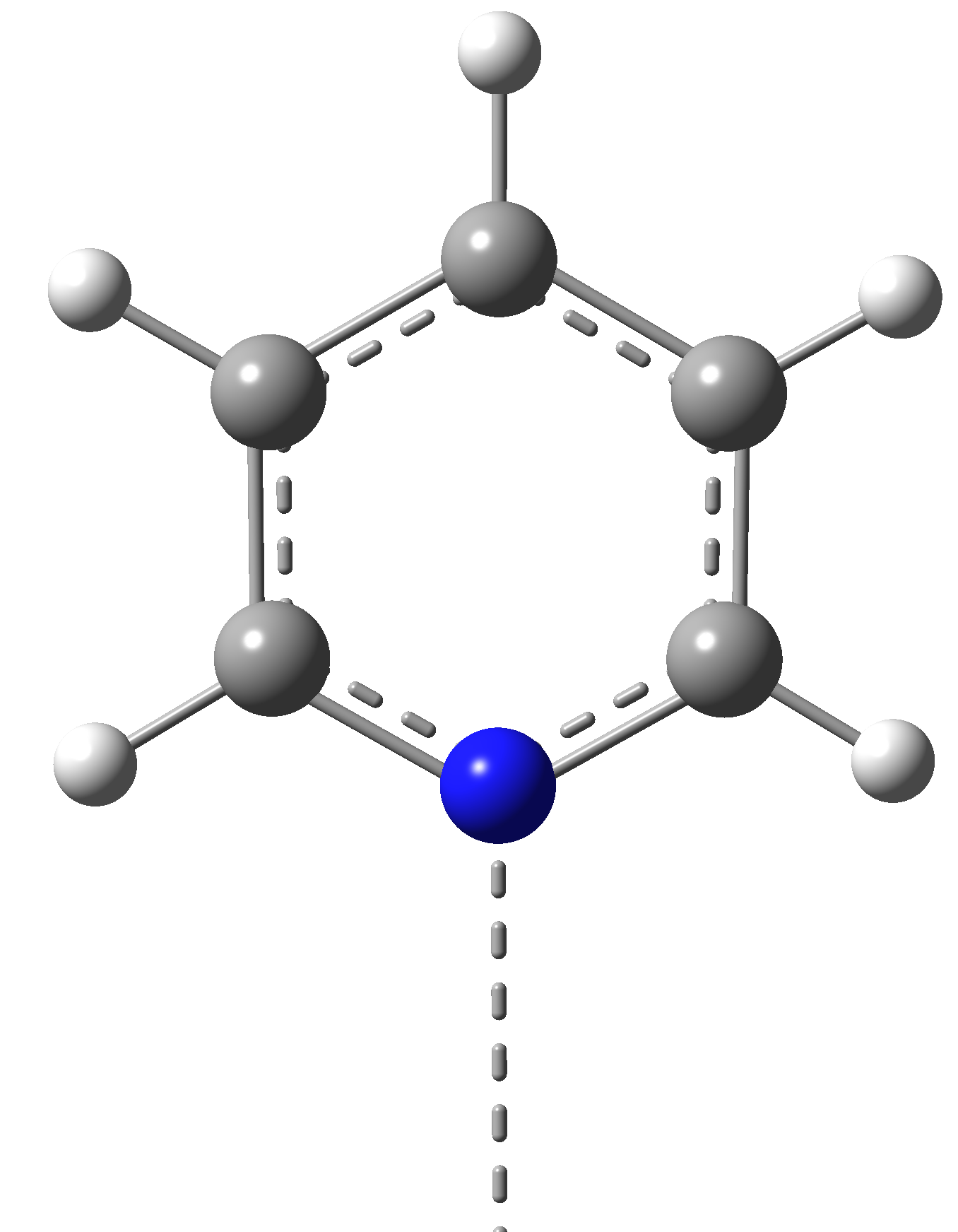
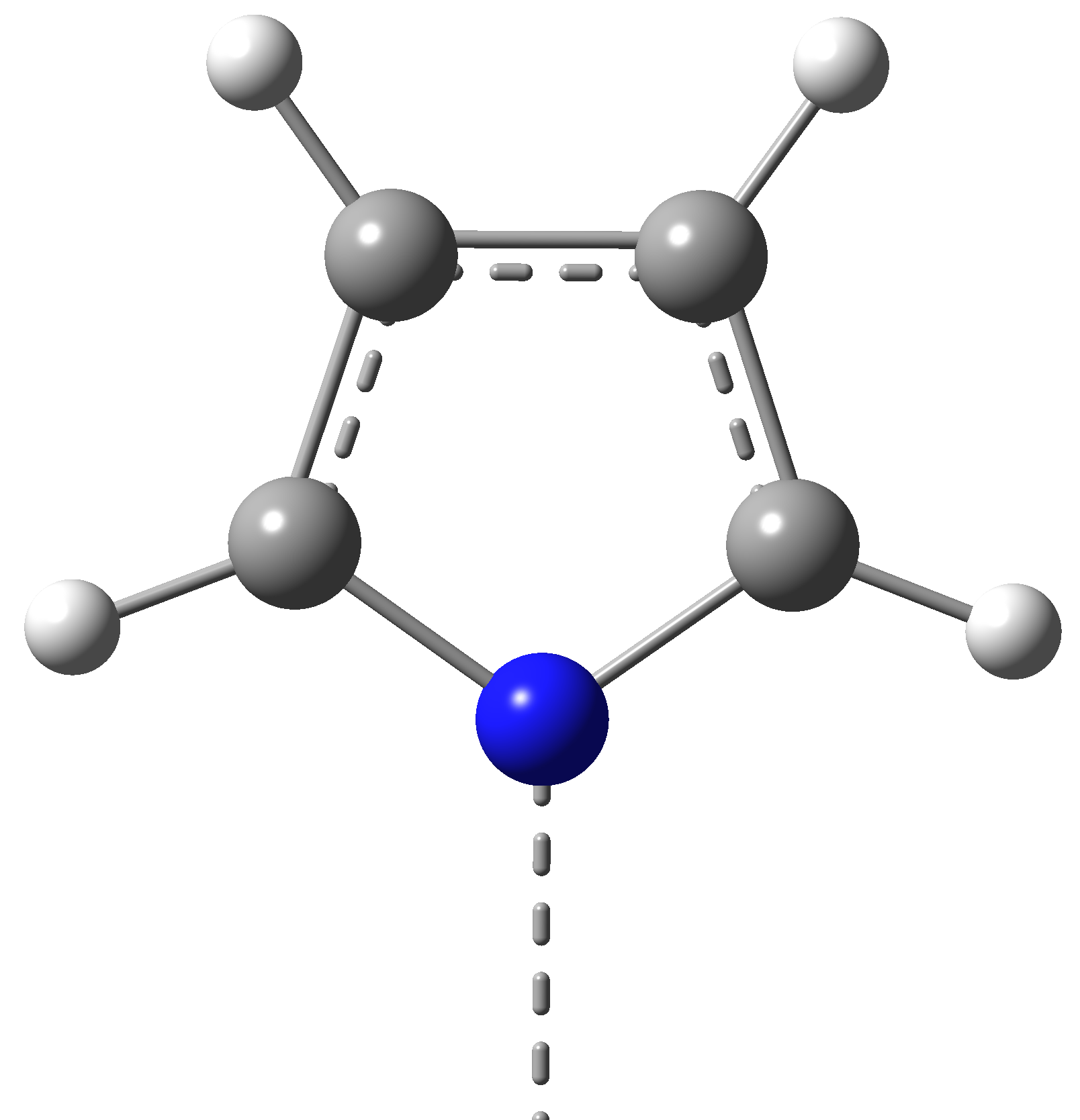
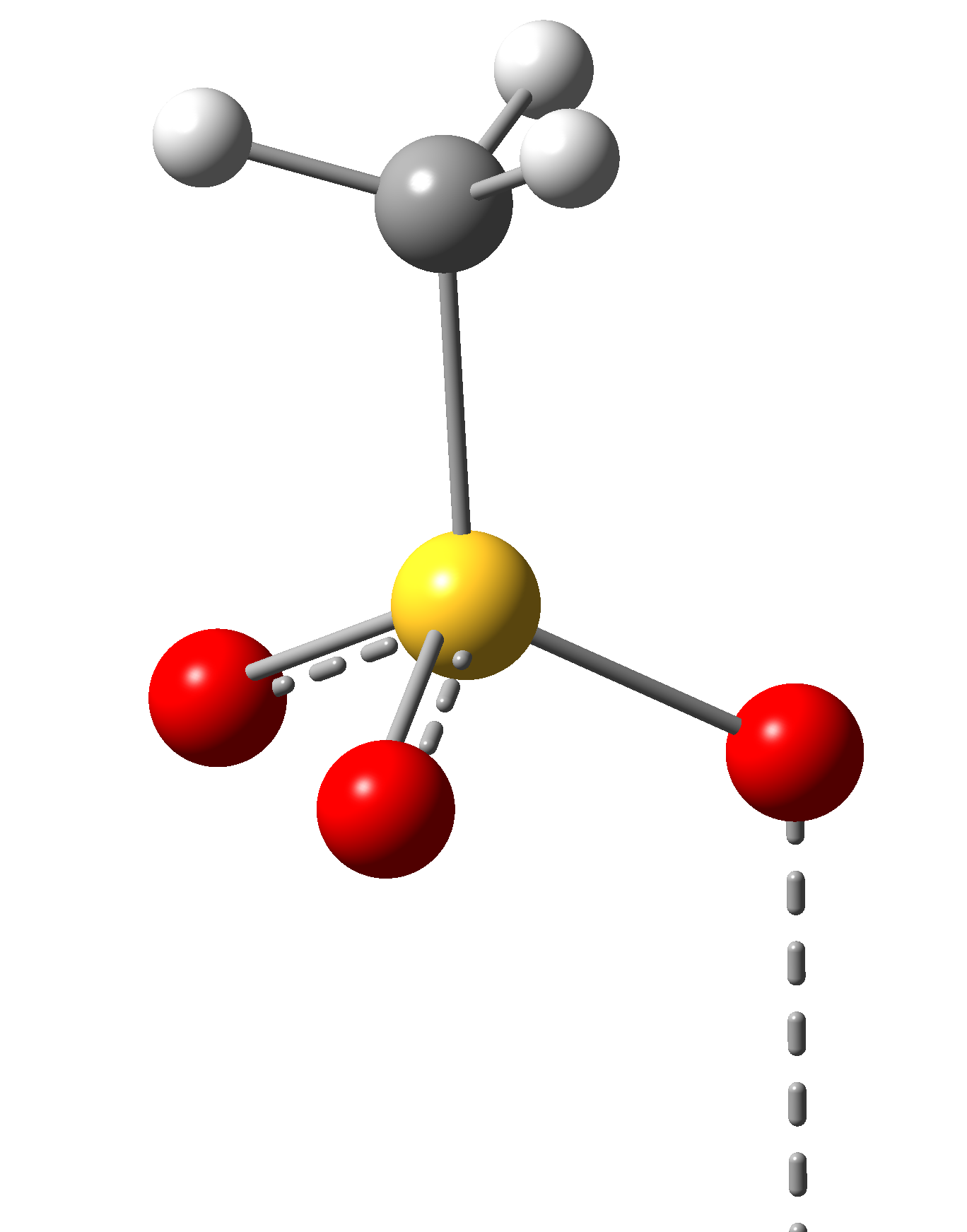
(Pi)
(Py)
(Me)
Figure 2. The vertical ligand fragments (Pi = pyridine; Me = mesylate; Py = Pyrrole) in the square-planar and octahedral Ni(II) macrocyclic ligand complexes.
1.1 Metal-ligand coordination geometries
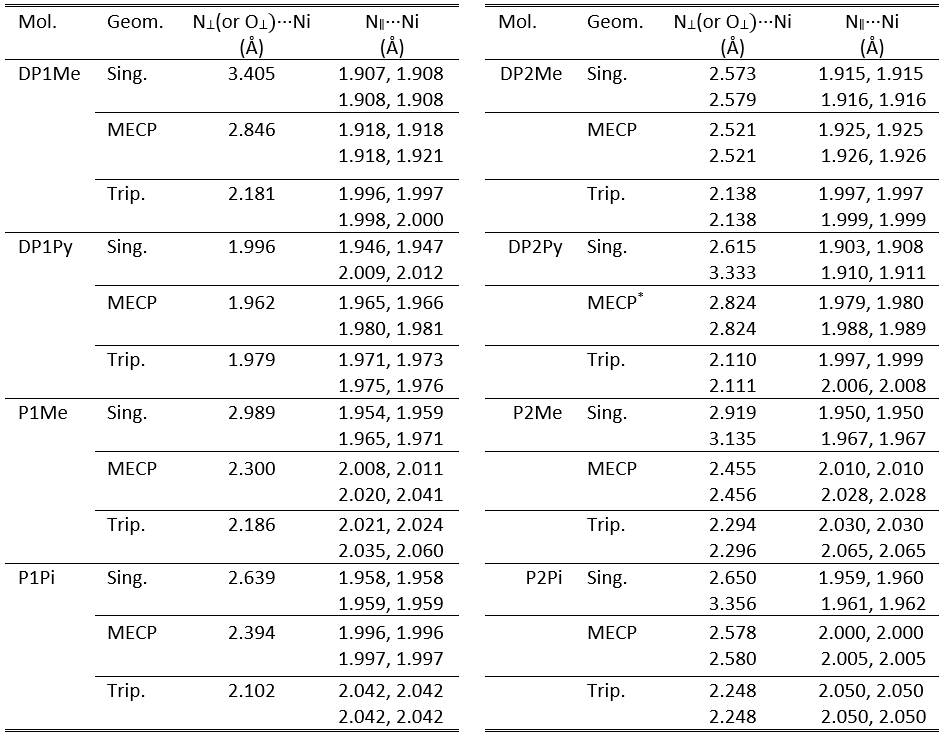
Several organometallic systems with square-pyramidal and octahedral coordination of XnY-type were constructed, where X denotes the four-coordination planar ligand (P or DP), n is the number of vertical ligands (1 – for square-pyramidal and 2 – for octahedral case) and Y means the type of vertical ligands (Pi, Py or Me). The equilibrium structures for the eight proposed organometallic geometries (DP1Me, DP1Py, P1Me, P1Pi, DP2Me, DP2Py, P2Me and P2Pi) considering both the singlet and triplet spin configurations were optimized at M06/def2-TZVP levels of theory. The bond lengths of planar (N∥···Ni) and vertical (N⊥(or O⊥)···Ni) ligands, as well as the relative conformational energy values are presented in Table 1.
Table 1. The characteristic ligand bond distances (in Å) between the Ni central atom and the nitrogen atoms for singlet and triplet spin configurations of the eight proposed organometallic complexes obtained at M06/def2-TZVP level of theory.
|
Geometry optimization calculations have shown that each metal-ligand complex has two, energetically stable conformations, one of them is typical for the singlet spin configuration, while the second one is characteristic for the triplet spin configuration. The most important difference between the two geometry conformations is the length of the vertical ligand bond, namely, in the triplet case, this bond length is always shorter than in the singlet case.
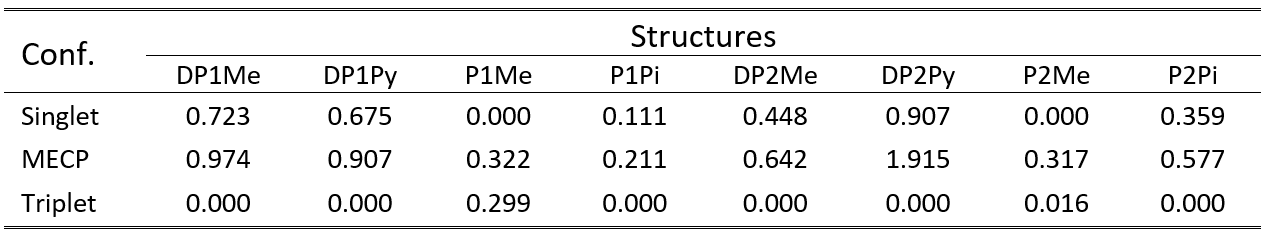
Table 2. The relative adiabatic energies for the singlet, MECP and triplet geometry configurations calculated for the eight proposed organometallic complexes at M06/def2-TZVP level of theory.
|
From energetical point of view, all cases show double-well potential energy profiles for the adiabatic singlet-triplet energy gaps (See Table 2). Namely, there are two energy minima for the equilibrium geometry of the singlet and triplet spin states and between them an energy barrier defined by the MECP geometry configuration. In six cases the lowest energy minimum is obtained for the triplet equilibrium geometry, while in two cases (P1Me and P2Me) the singlet spin state gives the most bounded conformations.
1.2 Thermodynamic effects
It is also known that external perturbations like thermal effects can considerably change the adiabatic energy gap calculated at 0 K by the so-called enthalpy-entropy compensation effects8,9. The normal mode vibrational analysis was performed using the M06 XC functional for both the low- and high-spin cases of the DP2Me metal-organic complex, and the thermally corrected adiabatic energy gap was computed. The reaction enthalpy effects enlarged the gap with about 0.53 eV, while the entropy effects were almost negligible (reduced the gap with about 0.006 eV). For the M06 functional, they finally gave an adiabatic LS-HS gap of 0.97 eV.
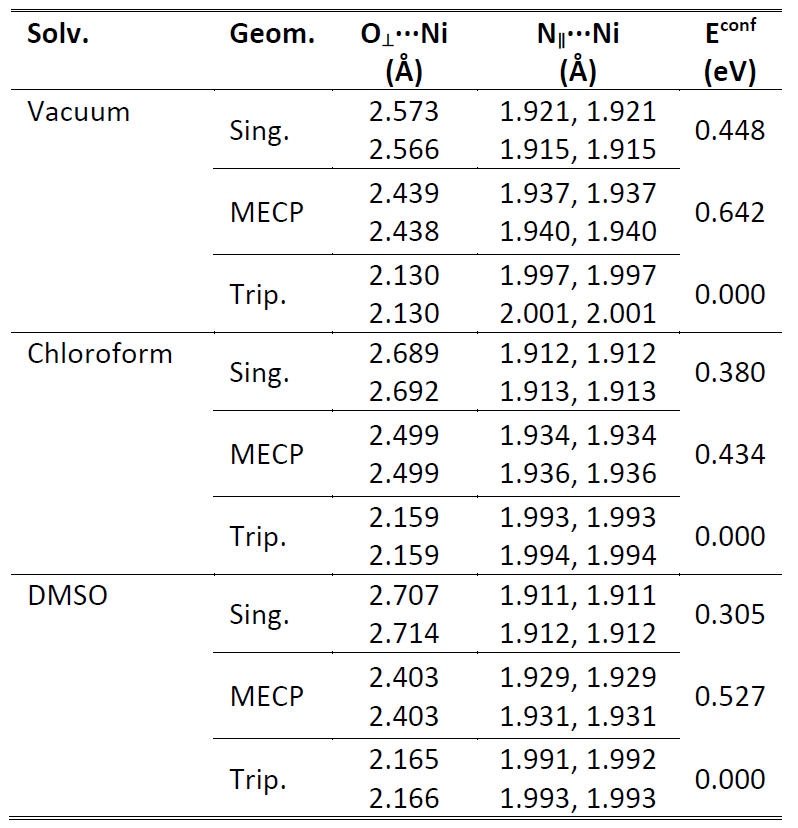
It has also been observed that the nature of the solvent can seriously influence the SCO characteristics of metal-organic supramolecular complexes10. Accordingly, two solvents (chloroform and DMSO) with different solvent properties were considered in order to follow the influence of the environment on the adiabatic HS-LS energy gap. The characteristic ligand bond distances and conformational energy differences for the singlet and triplet spin configurations, as well as for the MECP geometries calculated in non-polar (chloroform, ε = 4.8) and aprotic polar (DMSO, ε = 47.2) solvent environments, are given in Table 3.
Table 3. The characteristic ligand bond distances (in Å) between the Ni(II) central atom and the oxygen or nitrogen atoms for singlet and triplet spin configurations as well as for the minimum energy crossing point (MECP) geometries of the ground state singlet and triplet energies of the DP2Me metal-organic complex obtained at M06/CPCM/def2-TZVP level of theory considering two different solvents. The relative conformational energies (Econf in eV) between singlet, triplet, and MECP geometries are also given in the last column.
|
The results obtained at M06/CPCM/def2-TZVP level of theory showed that the influence of the solvent environments revealed a reduction of the adiabatic HS-LS energy gap, from 0.448 eV in vacuum to 0.380 eV in chloroform and 0.308 eV in DMSO, respectively. The energy barrier defined by the MECP geometry also decreased from the 0.642 eV in vacuum to 0.434 eV in chloroform and 0.527 eV in DMSO, respectively, as compared to the reference energy level of the triplet spin state. In summary, it could be said that solvent effects did not substantially change the relative positions of the HS-LS energy levels, they shifted down a bit the energies both for the singlet spin state and for the barrier between the singlet and triplet spin states as compared to the reference energy level12.
O3 - |
Chemical synthesis as well as structural and dynamical characterization of the designed molecular complexes; |
M07 - |
Continuing the chemical synthesis in case of the most performant spin crossover complexes and characterize them using UV-Vis, fluorescence, transient absorption and Raman spectroscopy techniques; |
M08 - |
Structural characterization the metal-coordinated macrocycles and embedding them in sulfonated coordination polymer networks; |
2. Chemical synthesis of the proposed SCO complexes
2.1 Chemical synthesis of di-keto pyrphyrin macrocycle
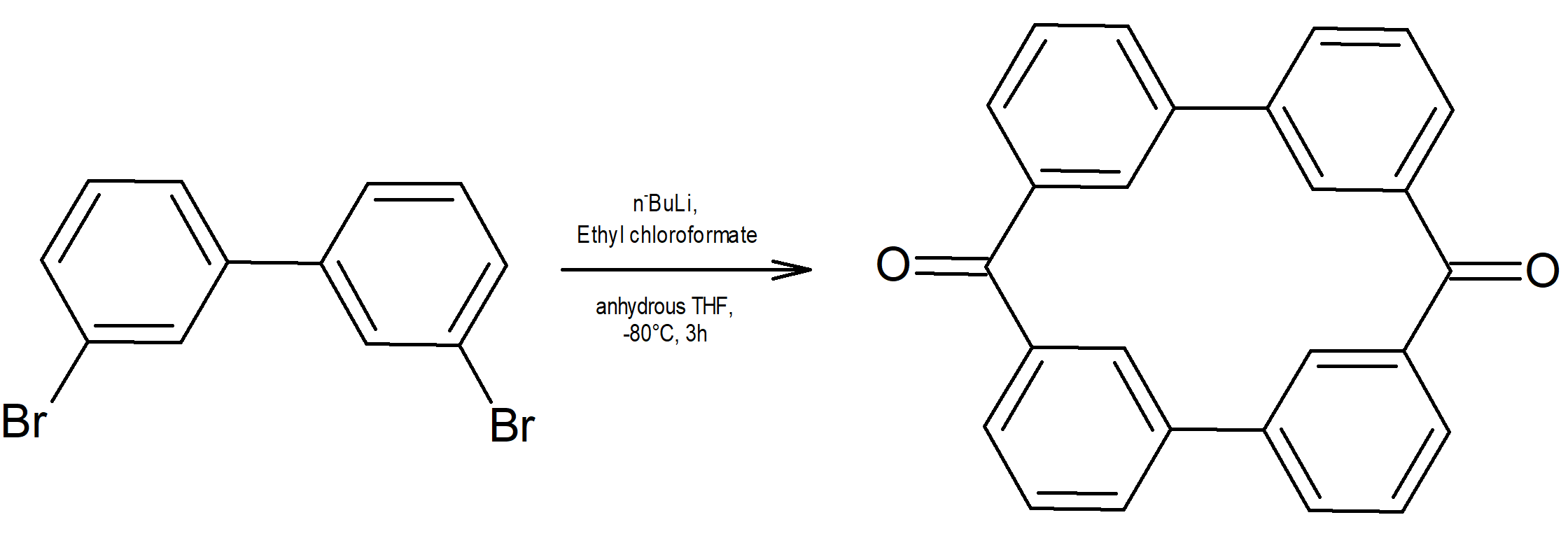
The chemical synthesis of di-keto pyrphyrin macrocycle was performed based on the procedure reported by Joliat-Wick et al12. The synthesis scheme is presented in Figure 3.
|
Figure 3. The synthesis scheme of the di-keto pyrphyrin macrocycle (nBuLi = n-butil lithium; THF = tetrahydrofuran).
Di-keto pyrphyrin was synthesized but it could not be purified; the macrocycle is not stable even if it has been stored at low temperatures. It will repeat its synthesis but the reaction mixture will be carried out in an inert atmosphere. The reaction products will be stored under argon and immediately after the spectral confirmation of the macrocycle obtaining, complexing reactions with nickel, manganese and iron (II) will be carried out in order to obtain the target polymers.
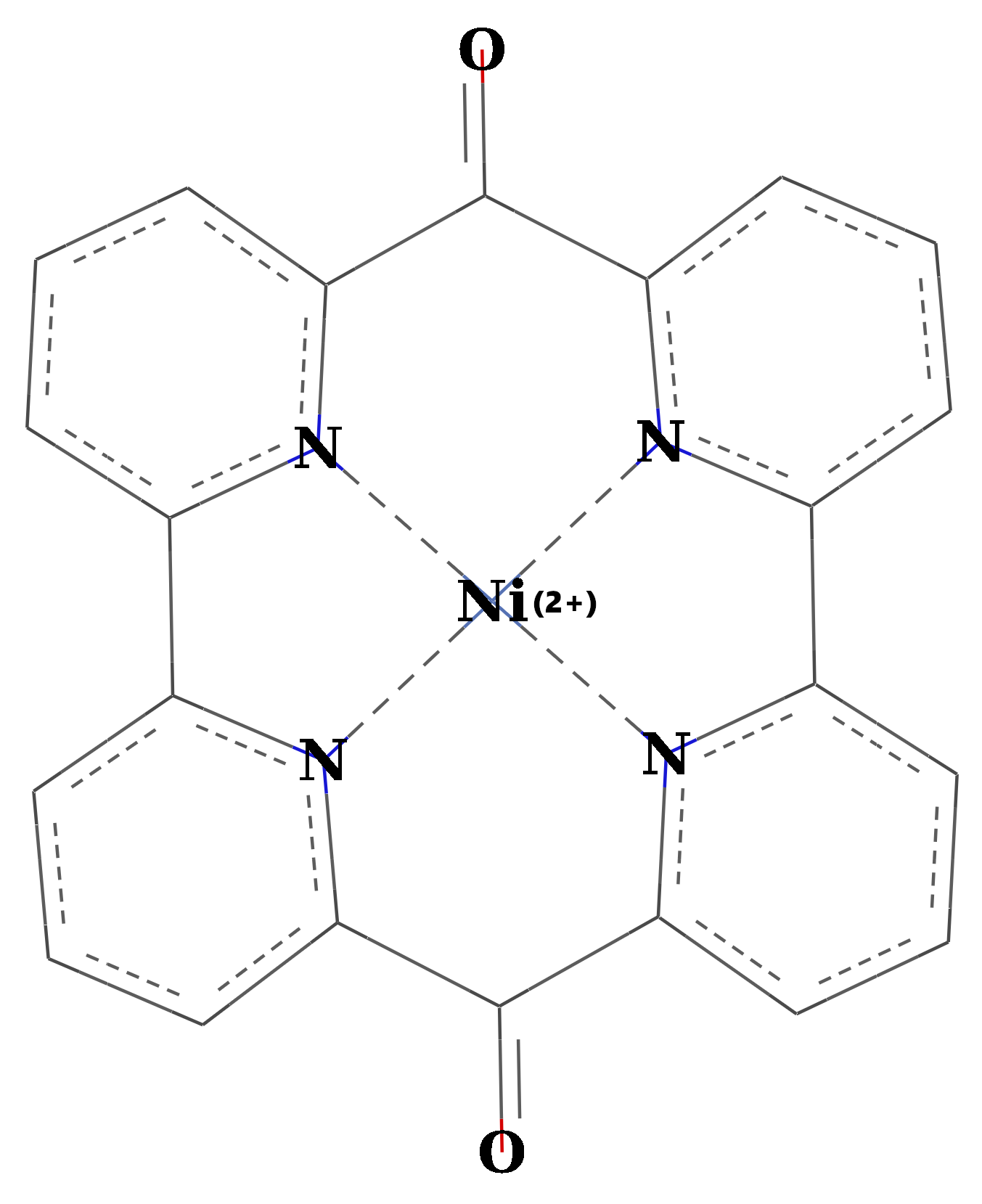
2.2 Complexation of Ni-Phthalocyanine
Experiments were carried out for fabrication and structural characterization of some phthalocyanine nickel (II) adducts with triflic acid (TrifA), trifluoroacetic acid (TrifAcetic), sodium mesylate (MesNa) and potassium mesylate (MesK) respectively.
|
Figure 4. The chemical structure of Ni(II)-Phthalocyanine (PhNi).
Following the adduction experiments of the phthalocyanine nickel (II) complex, a new compound was obtained in the case of the PhNi-TrifA2 experiment. Its chemical structure is under investigations by mass spectrometry and NMR on solids techniques. It will also try to crystallize it in order to determine the molecular structure by X-ray diffraction on a single crystal.
2.3 Selecting the excitation frequencies of the laser source
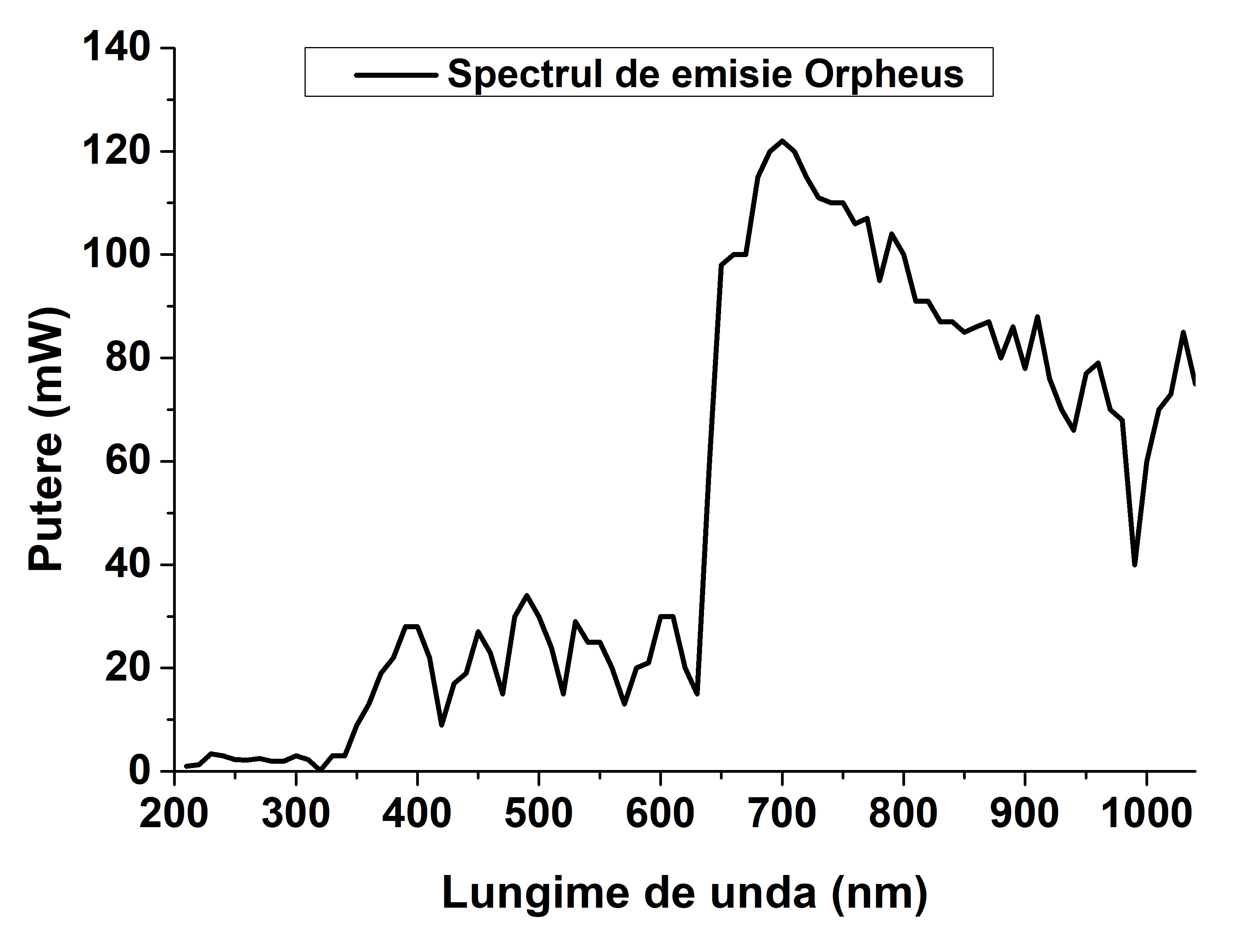
The identification and selection of laser excitation frequencies with good radiation absorption efficiency are very important. To this end, we conducted a detailed investigation on the technical parameters of the experimental device (laser radiation source) in order to compare with the absorption frequencies obtained based on theoretical studies regarding the absorption efficiency of the proposed organometallic complexes for chemical synthesis. The emission spectrum of the experimental apparatus is shown in Figure 5. As can be seen from Figure 5 the high laser radiation intensity spectral domain can be obtained for the frequency region 630 -1100 nm, and the UV range between 330 - 630 nm shows a much lower intensity. From this point of view it is necessary to find technical solutions based on theoretical studies that allow us to obtain a higher laser power. From this point of view, two theoretical studies have been carried out within this project, aimed at finding optimal parameters of the laser by which the intensity of the emitted radiation can be adjusted13,14.
|
Figure 5. The emission spectrum according to the frequency range of the experimental apparatus.
Conclusions
✔
Through chemical synthesis, we have synthetized the compound called Struct_4 chosen by molecular design described in the 2018 report as one of the possible organometallic structures with spin-crossover type properties;
✔
Due to the fact that the initially proposed Struct_4 complex was broken down into the primary processing step of the reaction product, the variant with Phthalocyanine Nickel (II) was used as a planar ligand for which the complexed form was made with the mesylate-type vertical ligands;
✔
A theoretical study was carried out which proposed different schemes for the construction of ultra-short laser pulses in order to improve the radiation intensity in the UV spectral domain (330-630 nm);
✔
Theoretical investigations regarding the structural stability and the reversibility of spin transitions for planar ligands based on porphyrin and di-keto pyrphyrin were continued;
Bibliography:
H. Yersin and J. Strasser, "Triplets in metal-organic compounds. Chemical tunability of relaxation dynamics", Coord. Chem. Rev. 208, 331_364 (2000).
K. Kjaer, W. Zhang, R. Alonso-Mori, U. Bergmann, M. Chollet, R. Hadt, R. Hartsock, T. Harlang, T. Kroll, K. Kubicek, H. Lemke, H. Liang, Y. Liu, M. Nielsen, J. Robinson, E. Solomon, D. Sokaras, T. van Driel, T. Weng, D. Zhu, P. Persson, K. Warnmark, V. Sundstrom and K. Gaffney, "Ligand manipulation of charge transfer excited state relaxation and spin crossover in 354 [Fe(2,2 '- bipyridine)(2)(CN)(2)]", Struct. Dynam.-US, 4, 044030 (2017).
W. Zhang, K. Kjaer, R. Alonso-Mori, U. Bergmann, M. Chollet, L. Fredin, R. Hadt, R. Hartsock, T. Harlang, T. Kroll, K. Kubicek, H. Lemke, H. Liang, Y. Liu, M. Nielsen, P. Persson, J. Robinson, E. Solomon, Z. Sun, D. Sokaras, T. van Driel, T. Weng, D. Zhu, K. Warnmark, V. Sundstromb and K. Gaffney, "Manipulating charge transfer excited state relaxation and spin crossover in iron 357 coordination complexes with ligand substitution", Chem. Sci., 8, 515_523 (2017).
Y. Meng, O. Sato and T. Liu, "Manipulating Metal-to-Metal Charge Transfer for Materials with Switchable 359 Functionality", Angew. Chem., Int. Ed., 57, 12216_12226 (2018).
K. Kepp, "Consistent descriptions of metal-ligand bonds and spin-crossover in inorganic chemistry", Coord. Chem. Rev., 257, 196_209 (2013).
J. Khusnutdinova and D. Milstein, "Metal-Ligand Cooperation", Angew. Chem., Int. Ed., 54, 12236_12273 (2015).
H. Worner, C. Arrell, N. Banerji, A. Cannizzo, M. Chergui, A. Das, P. Hamm, U. Keller, P. Kraus, E. Liberatore, P. Lopez-Tarifa, M. Lucchini, M. Meuwly, C. Milne, J. Moser, U. Rothlisberger, G. Smolentsev, J. Teuscher, J. van Bokhoven, O. Wenger, "Charge migration and charge transfer in molecular systems", Struct. Dynam-US, 4, 061508 (2017).
O.S. Siig, K.P. Kepp, "Iron(II) and Iron(III) spin crossover: Toward an optimal density functional.", J. Phys. Chem. A, 122, 4208_4217 (2018).
K.P. Kepp, "Theoretical study of spin crossover in 30 iron complexes", Inorg. Chem., 55, 2717_2727 (2016).
W. Phonsri, P. Harding, L. Liu, S.G. Telfer, K.S. Murray, B. Moubaraki, T.M. Ross, G.N.L. Jameson, D.J. Harding, "Solvent modified spin crossover in an iron(III) complex: Phase changes and an exceptionally wide hysteresis", Chem. Sci., 8, 3949_3959 (2017).
A.-A. Farcaş and A. Bende, "Improving the light-induced spin transition efficiency in Ni(II)-based macrocyclic-ligand complexes", Molecules, 24(23), Art. №: 4249 (2019).
E. Joliat-Wick, N. Weder, D. Klose, C. Bachmann, B. Spingler, B. Probst, and R. Alberto, "Light-Induced H2 Evolution with a Macrocyclic Cobalt Diketo-Pyrphyrin as a Proton-Reducing Catalyst", Inorg. Chem., 57 1651_1655 (2018).
K. Kovács and V. Toşa, "Macroscopic attosecond chirp compensation", Optics Express, 27(15), Art. №: 21872 (2019).
K. Kovács and V. Toşa, "Three ways to select from two attosecond pulses", Sci. Rep., (2019). - submitted